By Terence Pyle
Instructions for use (IFUs) play an important role in the learning and mastery of complex medical devices
and must be carefully designed to support clinical workflows.
As medical devices have become more interconnected and complex, new demands have been placed on clinical workers to develop and maintain mastery of various medical devices. Complex health technology requires clinical users to understand, configure, operate, and troubleshoot medical devices. Devices that are difficult to learn or use invite harm and legal liability.
Complex medical devices require effective instructions for use (IFUs) to develop user proficiency and consistent proper use. Well-developed IFUs can play a valuable role in teaching end users how to operate a medical device safely and effectively. Poorly designed IFUs can be misused or ignored by healthcare professionals.
Using a systematic approach to determine necessary content and to develop, deliver, evaluate, and validate the IFU, helps to create effective instructional materials. Included in this article are tips for writing and designing medical device IFUs that facilitate learning. These recommendations cover both content and format. They include:
- Understanding the target audience
- Task analysis
- Developing content
- Writing warnings
- Testing the manual
Understanding the Target User
What information should be included in the IFU depends in part on who is using it and what level of knowledge they bring to the task. Identifying the characteristics of each user group is critical to creating an IFU that works for them. The primary users of the medical device might include a variety of healthcare professionals, such as physicians, nurses, and technicians.
Make sure to adjust the language to the relevant audience. If the users are physicians, consider using different language than if the medical device were intended to be used by technicians. Talking to people who use the medical device or devices is likely to determine general characteristics and context of use that could guide the best approach to writing instructions. What is their age? Are they elderly? Are they literate? At what reading level? Will the range of users understand the technical language used in the instructions? Will the user understand the circuit diagrams, tables, charts, and graphs used in the instructions?
Task Analysis
Task analysis is a collection of procedures for defining the content of an instructional unit. It defines the content necessary to solve a performance problem. During this process, the technical writer can view the content from the learner’s perspective; understanding the learner’s knowledge and background helps the technical writer determine the scope of the task analysis. The output of the task analysis is documentation of the content to be included in the IFU.
A procedural analysis is a specific type of task analysis for analyzing knowledge and tasks well suited for defining cognitive sequences involving a series of steps. It is conducted by walking through the steps with an SME and used to analyze tasks by identifying the steps required to complete them.
 To do a task analysis, walk through the steps using the actual equipment, identifying and arranging all the steps necessary for performing the entire process required to use the medical device. Walk through the steps using the actual equipment. The information gathered from the walkthrough will be developed into the IFU.
To do a task analysis, walk through the steps using the actual equipment, identifying and arranging all the steps necessary for performing the entire process required to use the medical device. Walk through the steps using the actual equipment. The information gathered from the walkthrough will be developed into the IFU.
Using Mental Models to Guide Content Development
A mental model is defined as the knowledge that the user has about how a system works, its component parts, the processes, their interactions, and how one component influences another. Mental models are useful in learning, retrieving and problem solving. Several studies have empirically tested the benefits of a mental model provided when used in learning and operating a device. In one study, Kieras and Bovair found that people who learned a mental model showed improvements in learning, recall, and transfer over those who did not.1 This study revealed that users having a mental model for the device improved performance on learning and retaining the operating procedures for a device.
Dick2 describes how users develop mental models by observing what happens with a process and drawing conclusions about how the process works. To develop instructional content explaining how to use a medical device, we provide users with information they can use to mentally re-create the same mental model we have for the process. A user’s ability to operate the device depends on how effectively the users can re-create and follow the mental model as described in the instructional content.
Mental models are a key concept in the development of user instructions. If we don’t help users develop accurate mental models of how a medical device is installed, operated, and proper troubleshooting of problems then users are left on their own to develop their mental models. If the user’s mental models are not correct, they may have problems setting up, configuring, and operating the device.
Developing the Content
Once the task analysis is complete, refine the instructions using appropriate language and graphics. As the instructions are refined, consider the user’s knowledge and expected use environment, uncovered during user research.
The instructions should focus on how to operate the device. The user needs straightforward information about what to do, how to do it, and when to do it. Do not bury this important information in a lot of text. Assume that the user does not have device or medical knowledge.
Provide logically ordered steps for the task and make the user aware of the importance of doing the steps in order. State the purpose and the expected outcome of each task. Tell the user what steps are essential, and which are optional. The language used should be written at a sixth to seventh grade reading level to ensure clarity. Many people will not reread something they do not understand.
Installing the Device
Installation information provides step-by-step instructions for putting the device into service. Provide procedures for initial start-up and for verifying that all system components operate properly.

Devices may require a check-out procedure for safety and effectiveness, including visual inspection or calibration. Identify when the check-out should be done, such as at the time of setup or before each use. Also, include step-by-step instructions for checking proper function of necessary device components. Explain what to do if the check-out shows that the device is not working properly. Provide guidance on who to call if there is a problem. Refer users to the troubleshooting section in the manual if any problems can be resolved without manufacturer intervention.
Operating Instructions
Provide a brief physical description of the device, its parts, and any ancillary accessory items. Include a graphic of the device showing all parts of the device such as buttons and displays.

Principles of operation provide conceptual information to enable the end user to perform tasks by understanding the purpose and the principles of operation of the device. Backinger3 et al suggests including the following information in the operating instructions:
- Special preparation the user needs before operation, such as handwashing or device warm-up procedures
- Warnings or safety instructions specifically related to operation should be placed immediately before the corresponding task or instruction
- Expected results of the correct and incorrect operation
- Importance of following operating steps in logical order to obtain the expected results
- Space for user-specific instructions
- Instructions on who to call if there is a problem
Troubleshooting the Device
Anticipate the problems the device users might have with the setup, operation, or maintenance of the device. Clearly and briefly describe the symptom of each problem so the user can easily match the description to the problem observed. If the device displays error messages, list them and what they mean. Explain the steps necessary to correct the problem.
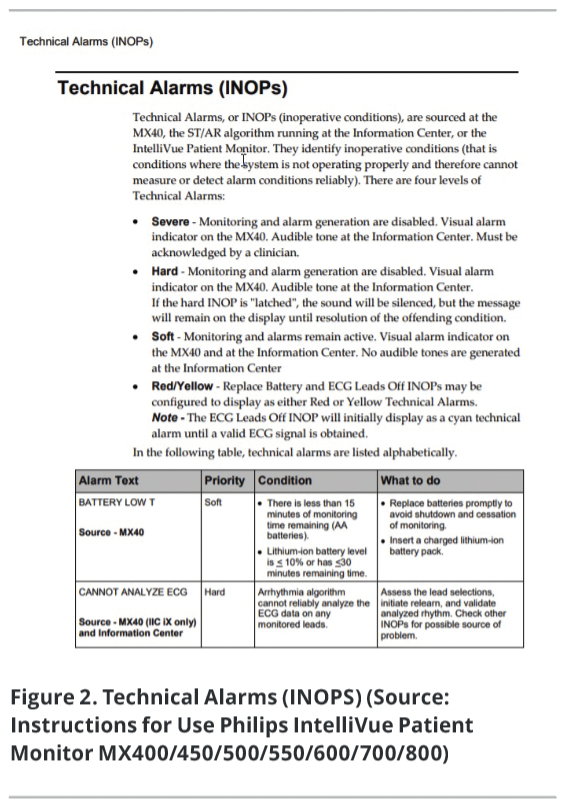
Do not confuse the reader with technical reasons for problems unless the reasons are important to the corrective action. If there are problems that users cannot or should not try to solve themselves, include warnings or cautions and tell them how to get help. Provide the user with guidance on how to report adverse incidents.
Written Warnings
Every IFU must identify all necessary warnings and precautions when using or preparing the device for use. Warnings and precautions identify harm or potential harm that can arise for the patient or end user because of device use and is rooted in risk assessment.
The ANSI Z535.6 standard4 gives detailed information on definitions, purpose, content, format, and location related to IFU warnings and precautions. Appendix E of the standard states that “research shows that hazard alerts are most effective when integrated into the task information at the most relevant location.”
General warnings and cautions provide critical information that should be heeded before using the device. Place all such warnings and cautions at the beginning of the manual where the user will see them right away.
A warning or caution that applies to a specific instruction or action should precede the instruction to which it applies. Researchers have found that a separate section combining all these types of warnings does not help the user avoid hazards unless these warnings are also included with the step in the instructions to which they apply. Include only warning information relevant to the upcoming procedural step.
IFU Outcome Measurements
Usability testing can help identify strengths and opportunities for improvement. Regulators consider IFUs to be part of the user interface of a medical device; they should be treated as an essential design element and tested in conjunction with usability tests of hardware and software components of a medical device.
It is important to conduct usability testing early and often. This way the effectiveness of the IFU is ensured and the guide is moving in the right direction. When performing usability testing for IFUs, there are several factors to consider. There are essentially three concepts that need to be evaluated as listed by Branaghan et al5:
- Search: Can people find and see the necessary information?
- Comprehension: Do people understand the information?
- Application: Do people perform the step appropriately?
Provide the IFU along with the medical device and direct the users to perform tasks. This approach will assess whether users spontaneously refer to the document and if the users find the document to be helpful.
Conclusion
Complex medical devices for patient care places new demands on clinical users to understand, set up, operate, and troubleshoot both stand-alone medical devices and integrated systems. Using principles from instructional design, technical writers can develop medical device IFUs that help their users develop a good mental model to learn how a device functions, how to troubleshoot it, and how to use it safely.
References
- Kieras, D.E., and S. Bovair. 1984. “The Role of a Mental Model in Learning to Operate a Device. Cognitive Science, no. 8, 255-273.
- Dick, David. 2014. “How to Design Documentation with Users in Mind.” Intercom 61, no. 7(July/August): 12-15.
- Backinger, C.L, and P.A. Kingsley. 1993. Write it Right: Recommendations for Developing User Instruction Manuals for Medical Devices Used in Home Health Care. Silver Spring: Department of Health & Human Services.
- ANSI Z535.6 2011 (R2017) For Product Safety Information in Product Manuals, Instructions, and Other Collateral Materials. Washington, DC: American National Standards Institute.
- Branaghan, R.J., O’Brian, J.S., Hildebrand, E.A., Foster, B. Humanizing Healthcare – Human Factors for Medical Device Design. Springer International Publishing, 2021.

TERENCE PYLE (pyleterence@gmail.com) is a senior technical writer at Comet Technologies where he collaborates with a group of Customer Quality engineers to write and edit process, procedures, and work instructions. He has worked in the field of technical communication for more than 30 years providing technical writing and editing services to manufacturers and system integrators of electronic systems. He earned his MS in Technical Communication from Colorado State University. Terence in his spare time enjoys researching his family history and meeting relatives at family reunions. He is a native Californian who has also lived in Colorado, Utah, and Wisconsin.




